-
Probing chiral recognition in liquid chromatography by absolute configuration modulation ATR-IR spectroscopy
R. Wirz, D. Ferri, T. Bürgi and A. Baiker
Spectroscopy Europe, 19 (1) (2007), p8-16


unige:14678
|
|
 |
|||||||
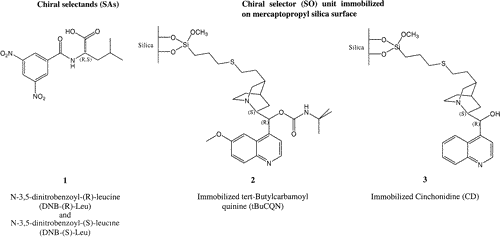
A method to selectively probe the different adsorption of enantiomers at chiral solid−liquid interfaces is applied, which combines attenuated total reflection infrared spectroscopy and modulation spectroscopy. The spectral changes on the surface are followed while the absolute configuration of the adsorbate is changed periodically. Demodulated spectra are calculated by performing a subsequent digital phase-sensitive data analysis. The method is sensitive solely to the difference of the interaction of the two enantiomers with the chiral surface, and the small spectral changes are amplified by the phase-sensitive data analysis. Its potential is demonstrated by investigating an already well-studied system in liquid chromatography, namely, the enantiomer separation of N-3,5-dinitrobenzoyl-(R,S)-leucine (DNB-(R,S)-Leu) using tert-butylcarbamoyl quinine (tBuCQN) as the chiral selector immobilized on the surface of porous silica particles. The performed experiments and density functional theory calculations confirm an interaction model that was proposed earlier based on solution NMR and XRD in the solid state. It emerges that the ionic interaction is the strongest one, but the main reason for the potential for enantioseparation of the chiral stationary phase (CSP) is the distinct formation of a hydrogen bond of the (S)-enantiomer with the chiral selector. This H-bond is established between the amide N−H of DNB-(S)-Leu with the carbamate C=O of the CSP. The (R)-enantiomer instead shows no specific hydrogen bonds. Only the unspecific ionic bonding between the protonated quinine part of the tBuCQN and the carboxylate of the DNB-(R)-Leu (holds also for DNB-(S)-Leu) is observed. | ||||||||
|
||||||||
A method to selectively probe the different adsorption of enantiomers at chiral solid−liquid interfaces is presented, which combines attenuated total reflection infrared spectroscopy and modulation spectroscopy. The weak spectral changes upon adsorption of enantiomers at a chiral interface are followed in time, while periodically changing the absolute configuration of the admitted chiral molecule. A subsequent digital phase-sensitive data analysis reveals spectral differences arising due to the different diastereomeric interactions of the two enantiomers with the chiral interface. The main advantage of the method compared to conventional difference spectroscopy is the enhanced signal-to-noise ratio. The method is selective for differences in diastereomeric interactions of the enantiomers. Its potential is demonstrated by studying the adsorption of ethyl lactate on a chiral stationary phase, which is amylose tris[(S)-α-methylbenzylcarbamate] coated onto silica gel. d-Ethyl lactate interacts stronger with the chiral stationary phase. In particular the spectral shifts reveal a stronger N−H···O=C hydrogen bonding interaction between amide group of the chiral stationary phase and the ester group of the ethyl lactate. The spectra also indicate that one of the three (S)-α-methylbenzylcarbamate side chains of the amylose derivative is predominantly involved in the interaction with the ethyl lactate. Furthermore, the experimental observations indicate that more than one interaction mode is populated at room temperature and that interaction with the ethyl lactate may induce a conformational change of the amide group of the chiral stationary phase. | ||||||||
|
||||||||
Alternative exposure of Pd thin films and Pd/TiO2 catalysts to dissolved hydrogen and oxygen leads to significant changes in the reflectivity of infrared radiation as observed in attenuated total reflection spectroscopy. The reflectivity decreases and the absorbance increases upon changing from oxygen- to hydrogen-saturated solvent. Reflectivity calculations based on the Drude model for the Pd thin film show that a slight change in the concentration of the free electrons of the metal could be at the origin of the observed effect. Alternatively, the reversible formation of a surface oxide layer can lead to a similar observation. The reflectivity changes can be used to follow the changes of the metal catalyst, similar to potential measurements, however without the need to work in conducting media. They can be correlated with the observation of adsorbed species and the formation of reaction products. The potential of the method for in situ studies of catalytic solid−liquid interfaces is demonstrated for the oxidation of 2-propanol and ethanol. Upon changing from reducing to oxidizing conditions, the observation of reaction products is slightly offset with respect to the observed reflectivity change in both cases, whereas the frequency of the CO vibration shifts at the same time as the reflectivity increases. | ||||||||
|
||||||||
A new ATR-IR cell was designed, and its performance was characterized by modulation excitation spectroscopy (MES). The new cell allows concentration modulation at relatively high frequency without unnecessary phase delay in the response. The response delay due to convection and diffusion was studied at different flow rates and modulation frequencies by experiments and simulations. The diffusion behavior of a small relatively fast-diffusing molecule, acetonitrile, was compared with that of a large slow-diffusing molecule, hemoglobin, in water. Experimentally, significant differences in their diffusion behavior were observed. The flow and diffusion behavior of the probe molecules was described using two different models, the diffusion layer model and the convection−diffusion model, and the theoretical results were compared with the experiments. The diffusion layer model allows estimating an effective diffusion layer thickness near the surface of the internal reflection element. However, the simulated response is significantly different from the experimental one. On the other hand, the convection−diffusion model describes the flow and diffusion behavior of the solute molecules with high accuracy. This work forms the basis for the investigation of chemical and physical kinetics such as surface reaction and diffusion by MES. It also suggests criteria for appropriate experimental conditions in ATR-IR MES experiments. | ||||||||